Dr. Jiajian Wang from Sun Yat-Sen University, The Chinese University of Hong Kong, the Chinese Academy of Sciences, and the Shenzhen Key Laboratory of Metabolic Health, describes a research paper he co-authored and published in Aging’s Volume 16, Issue 3, entitled, “Generating detailed intercellular communication patterns in psoriasis at the single-cell level using social networking, pattern recognition, and manifold learning methods to optimize treatment strategies.”
—
Behind the Study is a series of interviews with researchers discussing their aging-focused research published in Aging (Aging-US). Visit the Aging YouTube channel to watch video interviews with more of our outstanding authors.
—
Please introduce yourself and your institution/affiliation.
We exemplify a paradigm of collaborative basic research between scientists and medical professionals. I am honored to hold the position of Distinguished Assistant Researcher at the Precision Medicine Center and Children’s Medical Center of Sun Yat-sen University (Shenzhen), with a multidisciplinary background spanning genetics and computer science. I have at my disposal two biomedical computing platforms (large-scale servers), which greatly enhance my ability to undertake studies on the mechanisms of disease pathogenesis in collaboration with physicians from various specialties.
During our partnership with the Dermatology Department of Shenzhen Maternity & Child Healthcare Hospital, we identified that the department possesses an extensive collection of skin tissue histopathological slides, significantly facilitating our research endeavors. Consequently, our investigation commenced, grounded on the analysis of over a hundred skin samples, thereby leveraging these resources to advance our understanding of skin-related diseases.
Introduce your paper published in Aging (Aging-US). What were the most notable parts of your work or surprises experienced during your research process?
Psoriasis is a complex, multifaceted chronic inflammatory skin disorder involving a multitude of cell types. Employing a diverse array of analytical approaches including social network analysis, pattern recognition, and manifold learning, we have delineated the communication patterns among cells at the single-cell level in psoriasis, with a particular focus on the pivotal ligand-receptor interactions. This in-depth analysis aims to furnish more precise therapeutic interventions for targeted modulation. By integrating pharmacogenomic information, our team has pinpointed the regulatory roles of specific cell types within the internal regulatory networks that align with external communication patterns, thereby identifying potential therapeutic targets. Utilizing cutting-edge immunohistochemistry proteomics technology, the study corroborates the association between psoriasis’s single-cell communication patterns and clinical manifestations, offering a novel perspective on the disease’s pathogenesis.
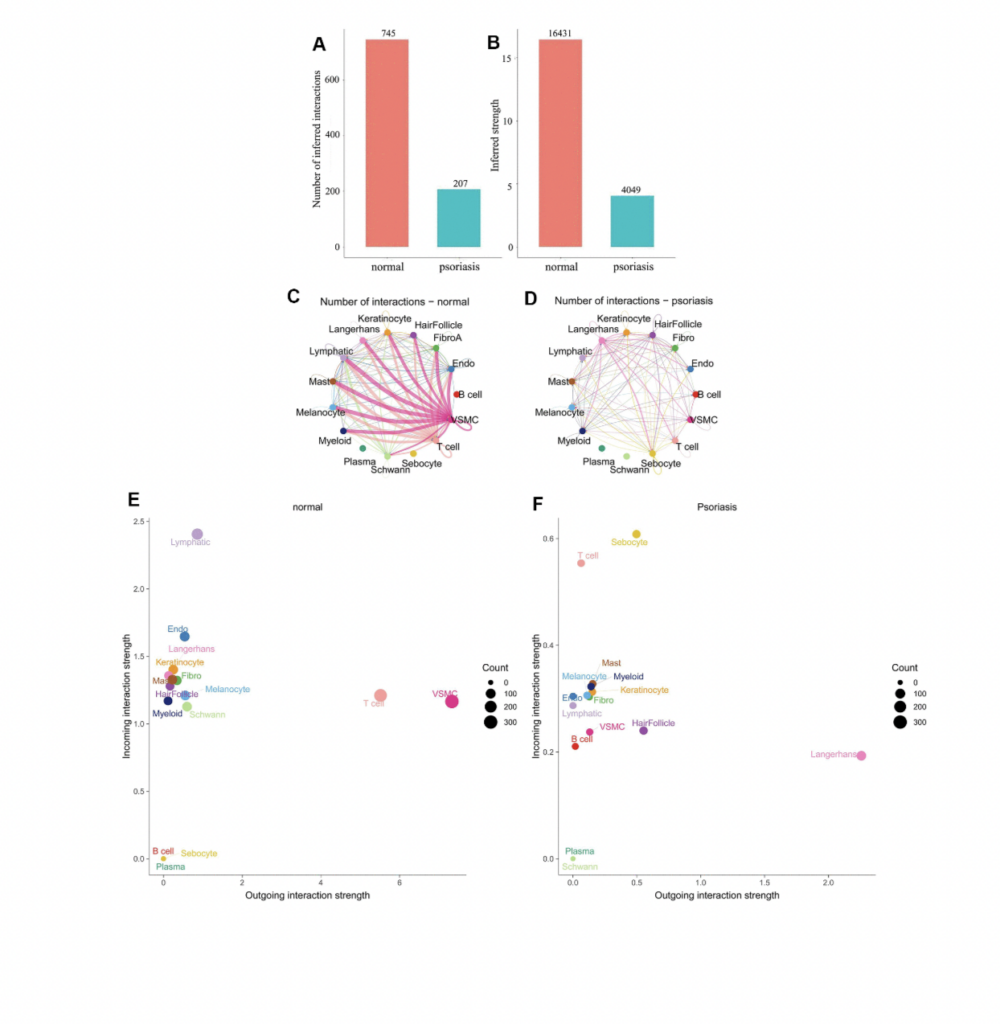
What are the key highlights of the study?
Synergistic Action of Immune Cells. Previous research has indicated an elevation of transitional B cells in the peripheral blood of psoriasis patients, possibly as a compensatory mechanism triggered by inflammation. However, our study reveals synergistic communication among immune cells in psoriasis, activating B cells as an input signal to jointly amplify the input signal of T cells. In contrast, in normal skin, T cells exhibit an output signal pattern, while B cell signals remain inactive. This marks the first report of synergistic communication between B cells and T cells at the single-cell level in psoriasis.
Critical Role of Langerhans Cells. As an essential component of the dendritic cell immunoregulatory network, Langerhans cells respond to antigen stimuli by migrating to local lymph nodes to present antigen peptides to T cells, thereby triggering specific immune responses. Our findings vividly display that in psoriasis, Langerhans cells dominate the output signal pattern through collagen and laminin signaling pathways. Beyond the synergistic action of immune cells, the study also discovers that hair follicle cells mediate communication between different cell types in psoriasis through the APP ligand, which is also linked to skin aging and the development of psoriasis inflammation.
Inhibition of Psoriasis Activation Pathways. Our research unveils that in psoriatic skin, many normal T cell signaling pathways (e.g., collagen, laminin, FN1, MPZ, and CD46) are significantly inhibited. Notably, only the CD99 signaling pathway remains consistently active, accompanied by the MIF signaling pathway. This suggests CD99 plays a foundational role in maintaining skin integrity, while the MIF signaling pathway becomes a key immune activation signal. Contrarily, B cells, predominantly utilizing CD99, take precedence in psoriasis, indicating a convergence of signaling pathways between these two immune cell types, potentially facilitating the transmission and feedback of immune signals.
Hair Follicle-Psoriasis Axis and Its Therapeutic Potential. Substantial evidence supports the hair follicle-psoriasis hypothesis. For instance, scalp psoriasis is associated with sebaceous gland atrophy. The hair follicle’s outer root sheath contains melanocytes and Langerhans cells and maintains close contact with resident immune cells (such as keratinocytes, lymphatic endothelial cells, and mast cells), crucial for the recruitment and activation of immune cells. Compared to immune cells surrounding the hair follicle, hair follicle cells appear to play a key role in immune regulation. Moreover, communication patterns suggest that the signaling mode of T cells in psoriasis shifts from an output to an input mode, likely influenced by signals emitted by resident immune cells associated with hair follicles.
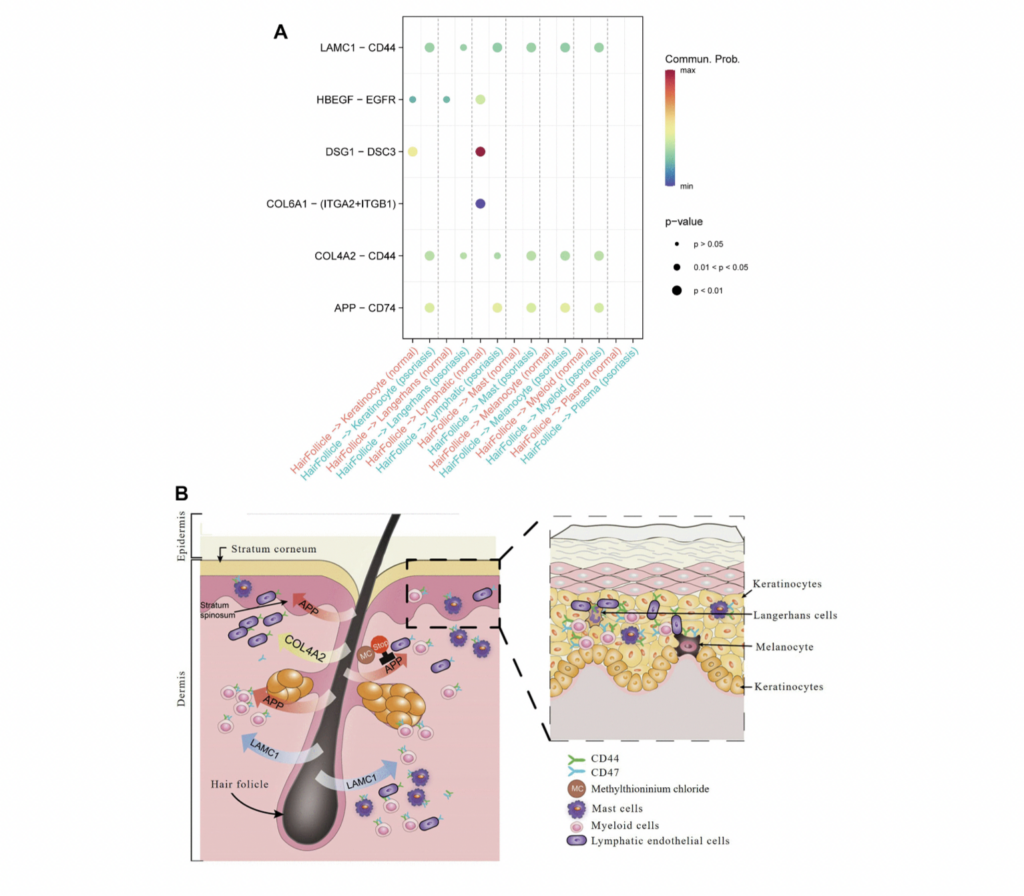
Therapeutic Potential. Traditional anti-psoriasis medications (e.g., methotrexate, corticosteroids, and cyclosporin A) need to be absorbed by skin cells to exert pharmacological effects. However, this study proposes an approach to enhance psoriasis treatment by inhibiting cell-to-cell signaling pathways leading to inflammation, such as APP. The study identifies six drugs targeting APP, already clinically used in other diseases, among which ferrous methanesulfonate shows potential efficacy in treating skin inflammation. This research provides robust support for further clinical trials in psoriasis treatment.
What made you want to take on this research?
The recovery from skin inflammation is a recurrent process that profoundly affects patients’ quality of life. This study aims to identify breakthroughs within the immunological microenvironment of psoriasis by targeting pivotal hub cells and transcription factors. The goal is to lay a solid foundation for the subsequent development of drugs, such as the exploration and research of key factors including the synthesis of artificial analogs that act as antagonists to obstruct the progression of inflammation. By focusing on the intricate network of immunological interactions within psoriasis, we endeavor to unveil novel therapeutic targets that can lead to more effective treatments. This approach not only promises to enhance our understanding of the pathogenesis of skin inflammation but also opens avenues for the development of innovative strategies to mitigate the impact of chronic inflammatory conditions on patients’ lives. Through meticulous investigation of the key components driving the inflammatory response, this research seeks to contribute significantly to the field of dermatological therapy, offering new hope for individuals suffering from persistent skin conditions.
What will you be doing next or what would you like to see done with your research?
Moving forward, our research focus will revolve around the spatial microenvironment of psoriasis, employing the newly developed spatial proteomics and spatial transcriptomics technologies. We aim to further investigate the spatial molecular patterns of different inflammatory cells and their potential therapeutic targets.
Acknowledgements?
We extend our gratitude to the Aging journal for their interest in our research and invite experts in dermatology or immunology to continue following our research developments. Here is the homepage for my research work: https://orcid.org/my-orcid?orcid=0000-0003-1766-0681. We are thankful to Y.X. for providing guidance on the clinical applications of our research, to B.W. for facilitating our computational analysis endeavors, and to B.W. for offering invaluable feedback and reviewing this manuscript. Special appreciation goes to S.D.L. for enhancing the quality of abstract images. Our research benefited from the high-performance computing capabilities of the Dell Precision 7920 Tower workstation.
Click here to read the full research paper published in Aging.
—
About Aging:
Launched in 2009, Aging publishes papers of general interest and biological significance in all fields of aging research and age-related diseases, including cancer—and now, with a special focus on COVID-19 vulnerability as an age-dependent syndrome. Topics in Aging go beyond traditional gerontology, including, but not limited to, cellular and molecular biology, human age-related diseases, pathology in model organisms, signal transduction pathways (e.g., p53, sirtuins, and PI-3K/AKT/mTOR, among others), and approaches to modulating these signaling pathways.
Please visit our website at www.Aging-US.com and connect with us:
- X
- YouTube
- Spotify, and available wherever you listen to podcasts
Click here to subscribe to Aging publication updates.
For media inquiries, please contact [email protected].
